This site contains information about clinical trials sponsored by Novo Nordisk. It is not intended to replace the advice of a healthcare professional and should not be construed as providing advice or making a recommendation. The information on this site should not be relied on as the basis for any decision or action. Only a physician can determine whether a specific product is correct for a particular patient. If you have questions regarding any information contained on this site you should consult a physician.
Information for you as a volunteer
The most important part of a clinical trial is the people who volunteer to participate. And, from start to finish, the safety and well-being of clinical trial participants is a top priority.
Before you begin
A clinical trial can only start after researchers determine that the study is scientifically justified and approval is granted by local ethics committees and health authorities.
Before you officially enroll, the research team will go over the details of the study, including how long it will last, and what procedures you will encounter. Agreeing to participate after this thorough explanation is called agreement to take part, and it’s a key step in the process of getting you started.
During the trial
During the trial, you’ll meet members of the research team, who will be available to you from start to finish. This team includes the investigator, another name for the doctor or scientist in charge of the entire trial. You will also have a lot of interaction with physicians and nurses who are there to guide you through the process.
After your experience
Once you complete a clinical trial, you should feel a sense of pride knowing that you played a big role in the advancement of a medical treatment. At the end of the trial, the trial team will examine the results, and you will have an opportunity to see what the doctors discovered. The results will also be published for the public to review, because this is a part of Novo Nordisk’s commitment to transparency.
Before your child or teenager is enrolled in a clinical study
A clinical study in children/teenagers can only start after researchers have determined that the study can be justified, and approval is granted by local ethics committees and health authorities.
Participating in a clinical study is a very personal decision for every family. Before your child or you as a teenager is enrolled in a study, the study staff will explain the details of the study to both you and your parents. The information will among other include the treatment to be given, the procedures to be done and how long time the study will last.
Agreeing to participate after this thorough explanation is called agreement to take part and depending on the child’s age, both the child and the parents will need to sign an agreement to take part, before the child can start in the study.
During the study
During the study, you and your parents will meet members of the study team, who will be available to you from start to finish. This team includes study doctors and nurses, who will perform some of the procedures and support you and your family throughout the study.
When the child/teenager has finished the study
Every child/teenager and their parents, who complete a study should feel a sense of pride as you have played a big role in potentially getting new medication available for other children.
At the end of the study, the researchers will look at the results of the information collected. You and your parents will get an opportunity to see what was discovered in the study. The results of the study will also be made available for the public to review, as Novo Nordisk has a commitment to show the results of all the studies we conduct.
When you have finished the study, the study doctor will ensure that you are offered the best possible treatment.
Before your child or teenager is enrolled in a clinical study
A clinical study in children/teenagers can only start after researchers have determined that the study can be justified, and approval is granted by local ethics committees and health authorities.
Participating in a clinical study is a very personal decision for every family. Before your child or you as a teenager is enrolled in a study, the study staff will explain the details of the study to both you and your parents. The information will among other include the treatment to be given, the procedures to be done and how long time the study will last.
Agreeing to participate after this thorough explanation is called agreement to take part and depending on the child’s age, both the child and the parents will need to sign an agreement to take part, before the child can start in the study.
Privacy notice for use of personal data and human biosamples
Why we collect and use personal data and human biosamples
At Novo Nordisk we focus on bringing new innovative medicine to the market and make them available across the world. We do this to defeat diabetes and other serious chronic diseases to the benefit of patients.
To develop new medicine, we need to do research to learn more
about the disease. We collect infomration from patients and from
clinical studies. Some of this information is personal data.
Examples of personal data are:
- human biosamples (for example tissues, blood and blood derivatives such as plasma, serum and cells)
- data colleced during clinical studies or from external parties (for example results, information and discoveries) medical images (for example scanning, radiography and x-ray)
We use and collect the personal data in a responsible and ethical way in accordance with our internal values, external requirements e.g. good clinical practice, and the applicable laws and guidance. When doing clinical research, we ensure that the participants are well informed about the study before they decide if they want to take a part.
For some research activities, we need data that we cannot collect
ourselves. For this we may use data form external parties. We have
high standards when doing so. We make sure that all data can be used
for the intended purposes. We also carefully assess that the personal
data have been lawfully and ethically collected.
How we protect personal data and human biosamples
It is important to Novo Nordisk to respect your rights and to keep your personal data private. The Privacy Notice explains how we protect and process your personal data, including human biosamples. How we collect, use, store, delete and share your personal data will be explained in more detail in the Privacy Notice.
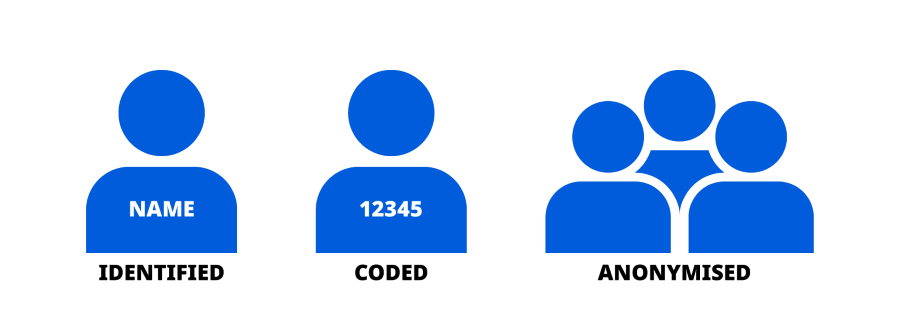
As a general rule, we will not be able to identify you directly in our research activities. We do this by replacing personal identifiers (for example, name or address) with a participant number. This means that the information is coded data. We will only try to identify you if there is a serious safety concern.
When we resuse data from our clinical studies for new scientifi
research purposes, we will try to use anonymised data, meaning that
there is no way to re-identify you.
Figure 1 below illustrates the different levels of masking personal data, where identified is personal data that has not been masked.
If It is not possible for us to use anonymised data for a specific
research activities, we may use coded personal data. The Privacy
Notice applies to search activites where Novo Nordisk uses
personal coded data and is not able to provide a notice of use
directly to you. An example could be where we get your health data
from hospitals, relatives, partners, suppliers or other external
parties. This could also be if we reuse already collected personal
data for new compatible research purposes.
"I have learned more about my disease from my 16 weeks of trial participation than I have during the past 26 years as a patient"
Participant experience
“It was a relationship you don’t get with most doctors – I felt confident every time I came”
Did you know?
Participant experience
Clinical trials are an irreplaceable step in the process to develop new medicines.
Participant experience
The results of all completed Novo Nordisk clinical trials are published.
Participant experience
Participants in a trial may be eligible for compensation or reimbursements.
Participant experience
You are welcome to join a clinical trial without a referral from your doctor.
Questions or concerns
Do you have any question about what it’s like being a clinical volunteer? Have a look at our answers to frequently asked questions.
Find trials in your country

